Ferrous Sulphate remove chromium
Ferrous Sulphate can be used as reducing agent to decrease chromate in cement. In the process of cement production, 900 ppm of ferrous sulphate can make hexavalent chromium (Cr6+) decrease to 2 ppm.
Chemical deposition:
Formula: H2Cr2O7+6FeSO4+6H2SO4=Cr2(SO4)3+3Fe 2(SO4)3+7H2O
Processes:
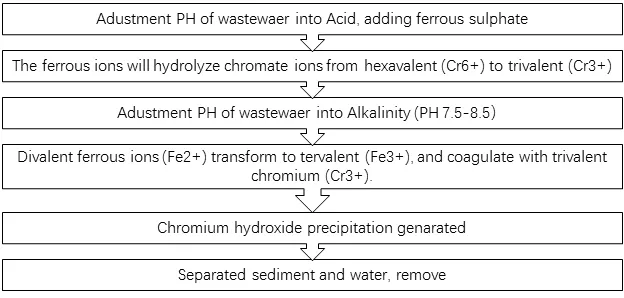
The harm of hexavalent chromium (Cr6+):
- Human skin and respiratory system will be harmed.
- Hexavalent chromium is also the dangerous carcinogens. Hexavalent chromium leaching out gradually from the cement, and dissolve in water, dissolving out for 7 months. After the rain, it will cause a large area of water pollution.
In California, hexavalent chromium be provision that cannot exceed 10 ppb. But it still 500 times of the 0.02 ppb recommended by The Office of Environmental Health Hazard Assessment (OEHHA).
The ROHS of European Union rules that the sum of Pb, Cd, Hg and Cr6+ in packing must less than 100 PPM.
